Antibiotics are among the most important discoveries of modern medicine and have saved millions of lives since the discovery of penicillin almost 100 years ago. Many diseases caused by bacterial infections – such as pneumonia, meningitis or septicaemia – are successfully treated with antibiotics. However, bacteria can develop resistance to antibiotics which then leaves doctors struggling to find effective treatments.
Particularly problematic are pathogens which develop multi-drug resistance and are unaffected by most antibiotics. This leads to severe disease progression in affected patients, often with a fatal outcome. Scientists all over the world are therefore engaged in the search for new antibiotics. Researchers at the University of Göttingen and the Max Planck Institute (MPI) for Biophysical Chemistry have now described a promising new approach involving “antivitamins” to develop new classes of antibiotics.
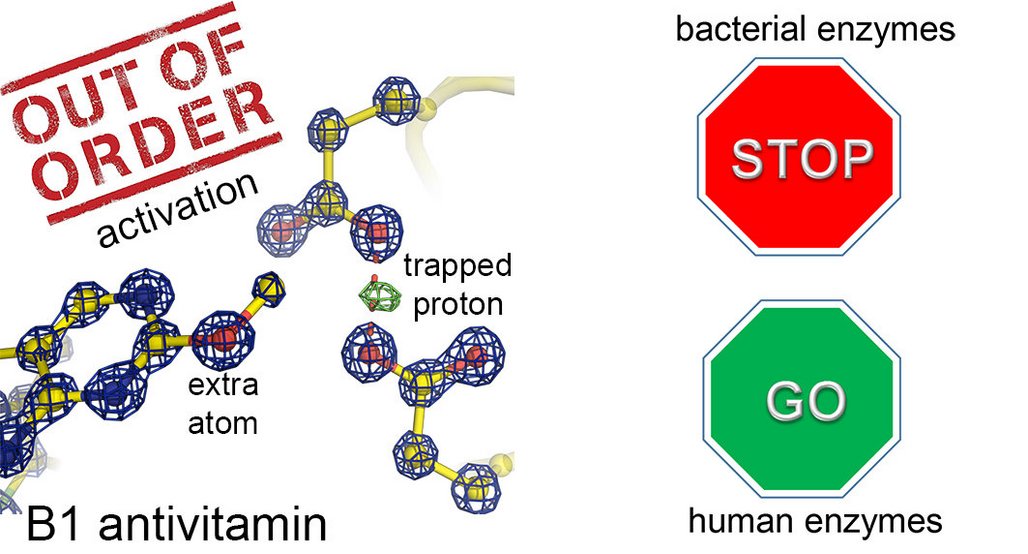
Antivitamins are substances that inhibit the biological function of a genuine vitamin. Some antivitamins have a similar chemical structure to those of the actual vitamin whose action they block or restrict. For this study, Kai Tittmann’s team from the Göttingen Center for Molecular Biosciences at the University of Göttingen worked together with Bert de Groot’s group at the MPI for Biophysical Chemistry and Tadhg Begley from Texas A&M University (USA). Together they investigated the mechanism of action at the atomic level of a naturally occurring antivitamin of vitamin B1. Some bacteria are able to produce a toxic form of this vital vitamin B1 to kill competing bacteria. This particular antivitamin has only a single atom in addition to the natural vitamin in a seemingly unimportant place and the exciting research question was why the action of the vitamin was still prevented or “poisoned”.
Tittmann's team used high-resolution protein crystallography to investigate how the antivitamin inhibits an important protein from the central metabolism of bacteria. The researchers found that the 'dance of the protons', which can normally be observed in functioning proteins, almost completely ceases to function and the protein no longer works. "Just one extra atom in the antivitamin acts like a grain of sand in a complex gear system by blocking its finely tuned mechanics," explains the structural biologist. It is interesting to note that human proteins are able to cope relatively well with the antivitamin and continue working. The chemist de Groot and his group used computer simulations to find out why this is so. "The human proteins either do not bind to the antivitamin at all or in such a way that they are not 'poisoned'," says the Max Planck researcher. The difference between the effects of the antivitamin on bacteria and on human proteins opens up the possibility of creating new therapeutic alternatives to for effective treatment of bacterial infections.
Contact
Prof. Dr. Kai Tittmann
Molecular Enzymology Group,
University of Göttingen
+49 551 39-177811
ktittma@...
Prof. Dr. Bert L. de Groot
Computational Biomolecular Dynamics Research Group,
Max Planck Institut for Biophysical Chemistry
+49 551 201-2308
bgroot@...
Dr. Carmen Rotte
Press Officer and Head of Public Relations,
Max Planck Institute for Biophysical Chemistry
+49 551 201-1304
Carmen.Rotte@...
Original Publication
Fabian Rabe von Pappenheim, Matteo Aldeghi, Brateen Shome, Tadhg Begley, Bert L. de Groot & Kai Tittmann
Structural basis for antibiotic action of the B1 antivitamin 2′-methoxy-thiamine.
Nature Chemical Biology, August 24, 2020.
DOI