Many scientific studies rely on fluorescent probes for highlighting specific structures in cells. For a long time, immunofluorescence of fixed cells and tissues dominated the field of fluorescence microscopy leading to the development of compatible fluorescent dyes. Recently, however, more attention is directed towards imaging of living cells. This imposes new restrictions on fluorescent dyes – they have to be cell-permeable and should not interact with other cellular components except the target.
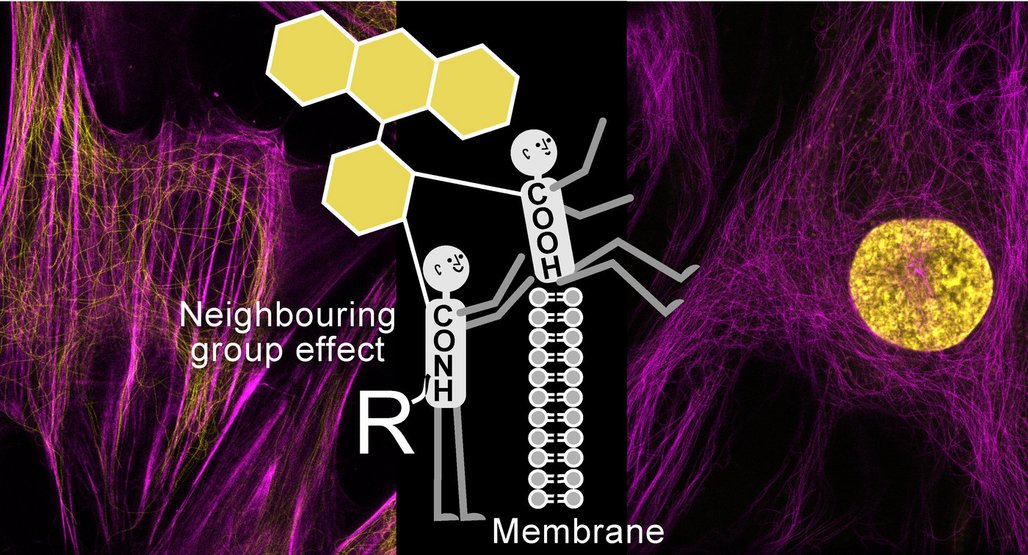
The researcher’s strategy was to enhance the cell permeability by exploiting the so-called neighboring group effect: A chemical group in a molecule influences the weak interactions in its immediate vicinity. Thereby, the chemical group also changes the way the molecule interacts with its environment. In this case, the scientists changed the position of a chemical group of a fluorescent dye in a way that the molecule can more easily penetrate the cell.
To do so, they used a well-known class of fluorescent dyes – rhodamines – which are known to exist as 5’- or 6’-carboxy isomers, depending on the position of the carboxyl group which forms the chemical bond between the fluorophore and the target-recognizing moiety. These classical isomers do not exhibit a neighboring group effect because the two carboxylic groups are separated in range. Lukinavičius and his co-workers introduced a new 4‘-isomer which has two carboxylic groups placed next to each other in the fluorophore molecule. „Such placement of two carboxyl groups close to each other in space activates a set of weak intramolecular forces, which changes the properties of the fluorophore, making it much more cell-permeable,” explains Jonas Bucevičius, first author of the article recently published in the journal Chemical Science.
“The most important feature of the 4’-isomer is that it can be applied to any rhodamine-like dyes, which experience difficulties with cell permeability. This broadens the toolbox of fluorescent dyes that can be used for generating new fluorescent probes,” biochemist Lukinavičius says. His team has patented the discovery which provides the scientific community with a new set of probes highlighting important cellular components.
Furthermore, until now, micromolar or high nanomolar concentrations of fluorescent probes were commonly used. The slight modification introduced by the research group decreases the working concentration by up to 100-fold. This has an additional benefit – removal of the fluorescent probe is not required after labeling. In addition, based on this principle, the Göttingen team also created probes for actin and DNA.
The rhodamine-like fluorophores exist in a hydrophilic zwitterion and a hydrophobic spirolactone form. The latter is non-fluorescent but it can penetrate cells. The equilibrium for the 5’/6’-isomers favors the less cell permeable zwitterion form, but introducing neighboring carboxylic groups in the 4’-isomer shifts this equilibrium towards the more cell permeable spirolactone form.
Contact
Dr. Gražvydas Lukinavičius
Head of the Research Group Chromatin Labeling and Imaging
+49 551 201-2540
grazvydas.lukinavicius@...
Original publication
Jonas Bucevičius, Georgij Kostiuk, Rūta Gerasimaitė, Tanja Gilat, Gražvydas Lukinavičius.
Enhancing biocompatibility of rhodamine fluorescent probes by a neighboring group effect.
Chemical Science.
Source
DOI