Researchers at the Max Planck Institute for Dynamics and Self-Organization show that two microscopic non-equilibrium chemically active particles, such as enzymes or catalytically active colloids, can have a vast range of complex interactions reminiscent of human relationships, instead of just simple attraction and simple repulsion.
Chemically active particles, such as enzymes or colloids, can propel in a liquid by converting chemical energy into mechanical work. A special characteristic of these particles is that they can violate Newton's third law: for a system of two particles, action and reaction are not necessarily equal, nor are they always in opposite directions. Despite this peculiarity, however, the relative interaction of these particles when they are in their simplest possible form, i.e. isotropic and equally sized, was previously thought to be straightforward: either purely attractive (in which case particles come together and form a complex), or purely repulsive (wherein the particles separate indefinitely), as in positive and negative charges in electrostatics. In a new paper, just published in Physical Review Letters, Babak Nasouri and Ramin Golestanian from the Department of Living Matter Physics at the Max Planck Institute for Dynamics and Self-Organization (MPIDS) show that the interaction between such particles through chemical signalling and modification of their surrounding liquid is much more complex.
Not just a simple attraction or repulsion: it’s complicated!
When it comes to studying the behaviour of these particles, the complexity of the underlying mechanisms often hinders thorough theoretical investigations and one has to resort to approximations. A widely used approximation scheme in this context that has so far informed our theoretical understanding of the behaviour of this system is the so-called 'far-field' approximation, which assumes that the distance between the particles is always considerably larger than their sizes. The far-field approximation predicts that the interaction between two such chemically active particles can be non-reciprocal, which is very non-intuitive, but despite that, it is always either purely attractive or repulsive, when gauged relatively. “Non-reciprocity of interactions is a rather remarkable feature that we are familiar with at the level of human interactions – e.g. B might like A but A might not like B – but is fundamentally new for microscopic particles, and is a manifestation of their non-equilibrium activity”, says Golestanian, director of the Department of Living Matter Physics at MPIDS.
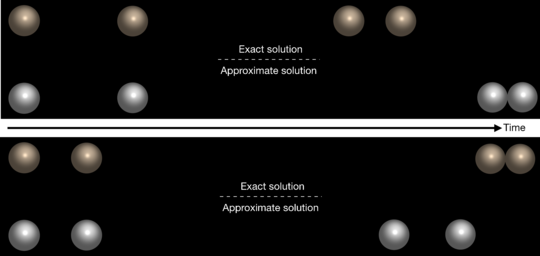
Although the widely used approximate far-field solution simplifies the governing equations quite significantly, in many cases, it can lead to incorrect predictions for the behaviour of the system. According to Nasouri and Golestanian, the relationship between two chemically active particles cannot always be categorized as purely attractive or repulsive. For example, the particles may move together as a stable bound-state with a constant nonzero equilibrium distance maintained between them. In this case, the particles repel each other if they get any closer than a certain distance, and attract each other if they separate any farther. They may also do the exact opposite, which can lead to the formation of an unstable complex that can be broken apart under sufficient perturbations or agitations. Nasouri and Golestanian show that the existence of these two new regimes of behaviour is due to the 'near-field' effects, which are completely ignored in the far-field description of the system. “It is very difficult to decide what can or cannot be ignored in modelling these complex systems,” says Nasouri, the first author of the study. “Approximate solutions are very useful for shaping our basic understanding of a system,” Golestanian adds, “but we cannot get the full picture without accurately, and if possible exactly, solving the governing equations.”
The underlying mechanism
Through an exact theoretical approach (which accounts for both near-field and far-field interactions), the authors show that the emergence of these two new regimes is due to a self-generated neighbour-reflected effect. When the particles are close to one another, each particle is affected by its own activity reflected back from its neighbour. “It is as if each particle acts as a mirror for its neighbour,” says Nasouri. He adds, “This reflection can work against the attraction or repulsion coming from the neighbouring particle, and that creates these new regimes of behaviour.”
Further information
Carolin Hoffrogge
Press officer
Phone: +49 551 5176-668
Email: presse@...