Researchers at the Max Planck Institute (MPI) for Biophysical Chemistry and the German Centre for Neurodegenerative Diseases (DZNE) have, for the first time, analyzed the fine structure of clumped alpha-synuclein proteins amplified from tissue samples from patients. Up to now, only artificially aggregated proteins had been investigated. The results show that the protein structures of clinical samples differ from artificial ones and are surprisingly diverse in Parkinson's patients. This shows how important supplementary studies with tissue samples are.
Arms and legs begin to tremble, muscles become stiff: Parkinson's and multisystem atrophy (MSA) are neurodegenerative diseases and can be accompanied by various impairments. They differ in their symptoms and also affect different parts of the nervous system. What both have in common, however, is that so-called alpha-synuclein proteins clump in the brain´s nerve cells and are deposited in various brain regions. “These deposits are a typical disease characteristic,” explains Markus Zweckstetter, research group leader at the MPI for Biophysical Chemistry and the DZNE.
The “normal” protein alpha-synuclein plays an important role in the communication of nerve cells. In addition to this variant, there are others that are associated with brain diseases such as Parkinson's and MSA.
A question of form
The typical fibrous deposits of alpha-synuclein are a possible starting point for drugs. The idea is that the active substances could prevent clumping or dissolve already existing aggregates. However, data on the fine structure of these protein accumulations is essential to identify possible docking sites. The question is: What form do the alpha-synuclein molecules take within the deposits?
“We asked ourselves how well the alpha-synuclein aggregates produced in the test tube reflect the situation in patients. That's why we looked at samples amplified from patients' tissue,” Zweckstetter says. “We worked closely with international partners on this project. The tissue samples came from Australia, the aggregates were amplified in South Korea. We carried out the structural investigations at the MPI for Biophysical Chemistry in Göttingen and at the DZNE.”
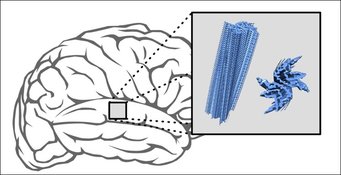
The scientists investigated brain samples from five deceased Parkinson's and MSA patients each. For comparison, the researchers artificially produced different variants of clumped alpha-synuclein aggregates. Using NMR spectroscopy and other methods, the scientists then compared the structure of the different aggregates.
Structural differences
“We found that the clumped proteins from the laboratory had a different structure than all deposits produced from patient material,” the study´s first author Timo Strohäker comments on the findings. “In addition, the proteins of MSA patients differed from those of Parkinson's patients. The proteins of the different MSA patients all had a largely similar form. The proteins of Parkinson's patients, in contrast, were much more heterogeneous.”
Various forms of deposits in Parkinson's
The different alpha-synuclein deposits in Parkinson's patients may explain why the course of Parkinson's disease can vary considerably from person to person. “This would contradict the hypothesis that Parkinson's disease is only associated with a single, clearly defined form of protein deposits. However, in view of our relatively small sample of five patients, this can currently only be assumed,” Zweckstetter says.
Contact
Prof. Dr. Markus Zweckstetter
Research Group Structure Determination of Proteins Using NMR, Group Leader at the German Center for Neurodegenerative Diseases (DZNE)
+49 551 201-2220
mzwecks@...
Carmen Rotte
Press Officer and Head of Public Relations
+49 55 1201-1304
carmen.rotte@...
Original Publication
Timo Strohäker, Byung Chul Jung, Shu-Hao Liou, Claudio O. Fernandez, Dietmar Riedel, Stefan Becker, Glenda M. Halliday, Marina Bennati, Woojin S. Kim, Seung-Jae Lee & Markus Zweckstetter
Structural heterogeneity of α-synuclein fibrils amplified from patient brain extracts
Nature Communications (2019)
DOI